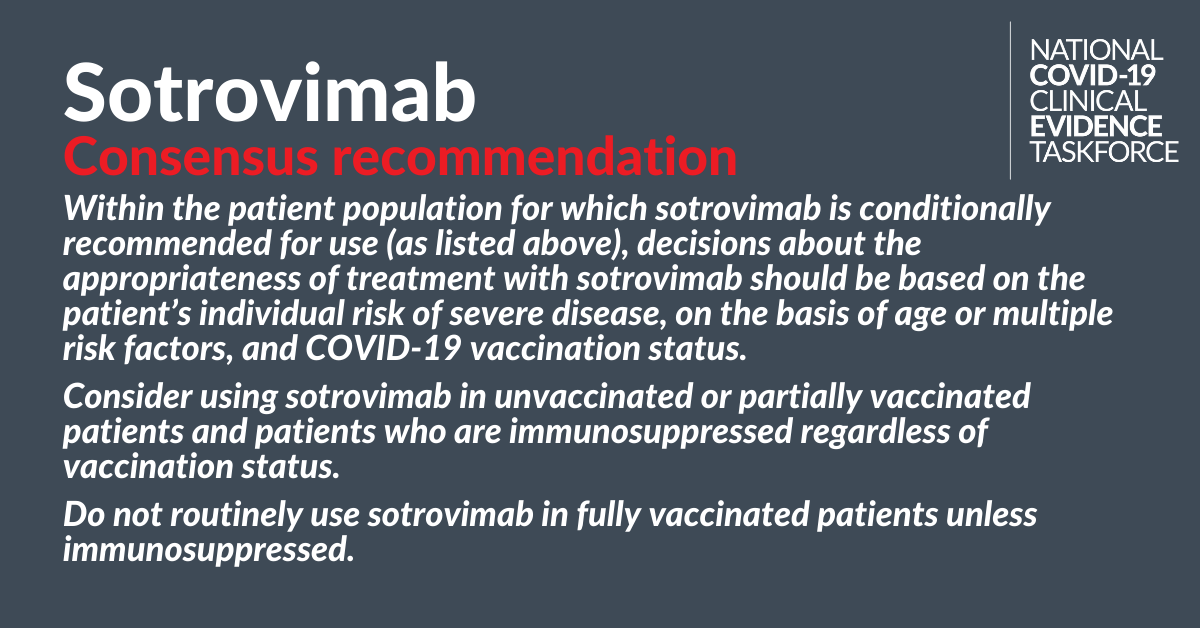
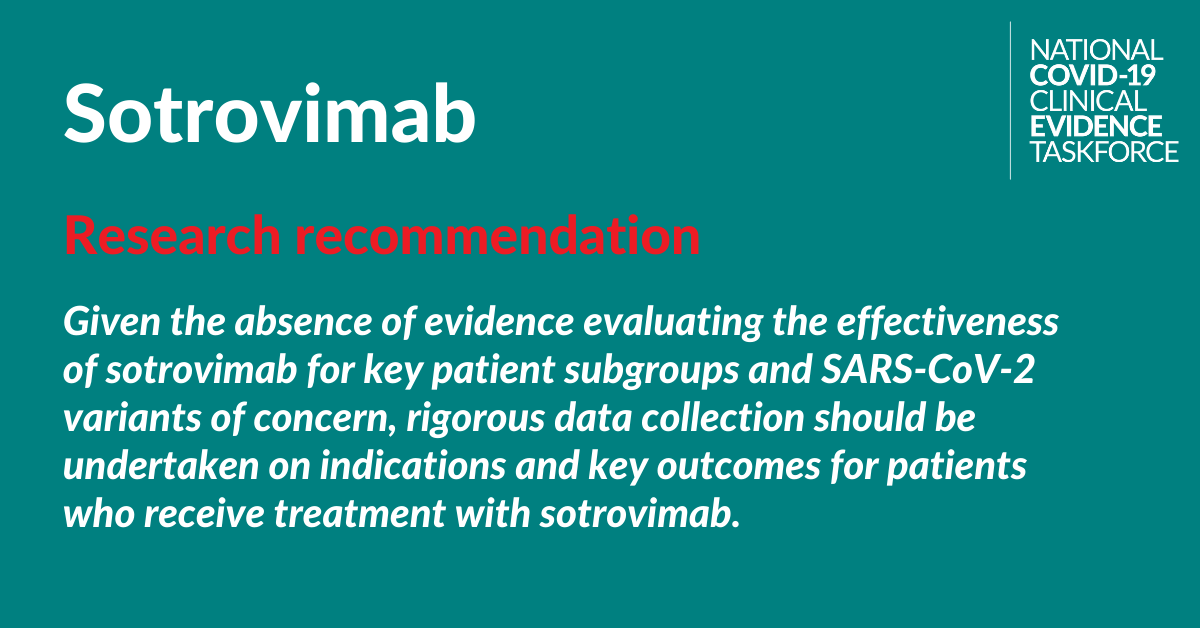
National COVID-19 Clinical Evidence Taskforce makes conditional recommendation for sotrovimab
The National COVID-19 Clinical Evidence Taskforce, of which the College is a member, has published three recommendations on the use of sotrovimab for the treatment of COVID-19 in adults who do not require oxygen and who have risk factors for disease progression. These are the first recommendations from the Taskforce to use a drug to treat patients with mild illness.

Seven new treatment recommendations for pregnant and breastfeeding women
Sotrovimab for pregnant women
The Taskforce has also reviewed the evidence in the context of pregnant and breastfeeding women. Three new recommendations have been developed for the use of sotrovimab in pregnant women, reflecting the adult recommendations.
Conditional recommendation: Supporting its use in pregnant patients within the second or third trimester who are at risk of disease progression.
Consensus recommendation: For pregnant patients that are unvaccinated/partially vaccinated/immunosuppressed.
Research recommendation: Regarding the need for rigorous data collection.
Casirivimab plus imdevimab (REGEN-COV) for pregnant or breastfeeding women
The Pregnancy and Perinatal Care Panel has also reviewed the casirivimab plus imdevimab for adults recommendation and have developed three new recommendations for pregnant and breastfeeding women that reflect the guidance for adult patients.
Conditional recommendation: Supporting its use in pregnant or breastfeeding patients that are seronegative.
Do not use recommendation: For pregnant or breastfeeding patients who are seropositive
Only in research recommendation: For mild or asymptomatic pregnant or breastfeeding patients.
New conditional recommendation for casirivimab plus imdevimab (REGEN-COV) for post-exposure prophylaxis
Following rhe Disease-Modifying Treatment and Chemoprophylaxis Panel review of the results of the COVID-19 Phase 3 Prevention Trial Team, the Taskforce recommends: Consider using subcutaneous casirivimab plus imdevimab as prophylaxis in seronegative or PCR-negative close household contacts of individuals with confirmed COVID-19.
Magnesium sulfate
The Pregnancy and Perinatal Care Panel have developed a consensus recommendation to support the use of magnesium sulphate as per usual care. The Panel determined that there are substantial benefits for using magnesium sulfate for fetal neuroprotection in preterm birth, and for the management of pre-eclampsia and eclampsia. There is currently no direct evidence to suggest additional harms of using magnesium sulfate for fetal neuroprotection in the setting of COVID-19.
New Respiratory and Eye Protection Decision Aid
As healthcare workers across Australia continue to face increased risk of exposure to SARS-CoV-2, the Taskforce Infection Prevention and Control Panel have developed a visual Decision Aid to help clinicians apply the PPE guidance in practice.
New immunodulatory treatments comparison table
The Taskforce has developed a comparison table of the recommended immunodulatory treatments (tocilizumab, baricitinib and sarilumab), including clinical and non-clinical information to help guide clinicians in selecting the most appropriate treatment for their patients.
Ivermectin FAQs
To support clinicians in providing evidence-based advice regarding the use of ivermectin as a treatment for COVID-19, the Taskforce has created a one-page pdf that covers our most frequently asked questions. This is available for download from the website here. The Taskforce recommends that ivermectin should only be used for the treatment for COVID-19 in the context of randomised trials with appropriate ethical approval.
Prone positioning for adults
One new trial has been added to the evidence base for prone positioning for adults. The consensus recommendation promoting the consideration of prone positioning for at least three hours per day as tolerated has been upgraded to a conditional recommendation.
Read the Taskforce's weekly communique here.

Get unlimited access to hundreds of ACP's top courses for your professional development.
Join Now